Synapse proteome
Neuronal networks underpin cognition in the brain. These are formed from the electrical neurons themselves and the chemical synapses that connect them. These chemical synapses translate electrical input from the presynaptic neuron and process it into a signal on the postsynaptic neuron that can stimulate, inhibit, or otherwise modulate the downstream connections depending on the nature and state of the dynamic protein signalling cascades located at the synapse. Critically, these chemical synapses are the key to understanding and treating brain diseases as they are the site of most genetic effects on neuronal function and they are the sites of most drug targets.
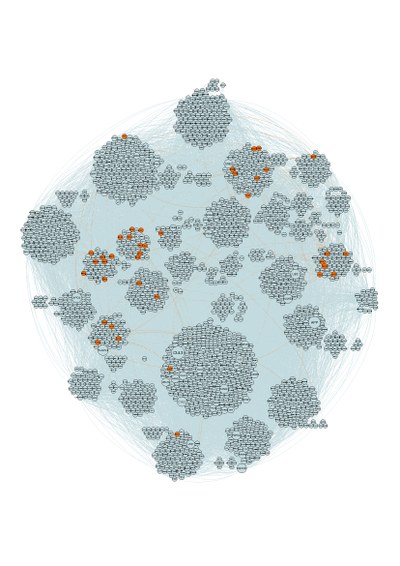
The synapse proteome describes the protein composition of neuronal synapses. Initiated by pioneering studies that identified 20-100 proteins in mouse excitatory synapses in the early 2000s a more complex picture has since emerged with numbers extending to over 8000 proteins (Sorokina et al., 2021) (~36% of the human genome), making it one of the most complex biomolecular structures known. This molecular complexity reflects the diverse array of processing tasks needed to underpin behaviours including sensory perception, motor control, learning and memory, which apply specific requirements on the molecular composition of each synapse.
There is a massive disconnect between the small numbers of molecules that are under active research by the scientific community and the 8000 potential candidates. We are assembling computational models (mostly employing on graph network methods) that can be used to study the architecture of the synaptic proteome (i.e. how the molecules interact with one another) and how this architecture correlates with the genetic fingerprint of neurological function and disease (i.e. large scale human genetics and animal model datasets). When combined, we can identify focussed clusters of highly interacting molecules with an enriched association with specific neuronal functions or with disease. These clusters provide new insights into disease mechanisms at the molecular level and an exciting source of radically new drug targets (e.g. Kanellopoulos et al., 2020)
Our approach is highly collaborative, and we are always interested in working closely with groups who have new proteomic, new disease association datasets or new computation methods they would like to explore.
People involved
Prof. Douglas Armstrong
Associated member
References
- Kanellopoulos, A. K., Mariano, V., Spinazzi, M., Woo, Y. J., McLean, C., Pech, U., Li, K. W., Armstrong, J. D., Giangrande, A., Callaerts, P., Smit, A. B., Abrahams, B. S., Fiala, A., Achsel, T., & Bagni, C. (2020). Aralar Sequesters GABA into Hyperactive Mitochondria, Causing Social Behavior Deficits. Cell, 180(6), 1178-1197.e20. https://doi.org/10.1016/j.cell.2020.02.044
- Sorokina, O., Mclean, C., Croning, M.D.R. et al. A unified resource and configurable model of the synapse proteome and its role in disease. Sci Rep 11, 9967 (2021). https://doi.org/10.1038/s41598-021-88945-7

