Olfactory receptors
Olfactory receptors (ORs) constitute the largest subfamily of class A G protein coupled receptors (GPCRs) including more than 400 proteins that are responsible for mediating the sense of smell. ORs allow the discrimination among more than 1 trillion different olfactory stimuli: one odorant can activate numerous types of ORs, while a single OR can be activated by several different odorants. These data clearly underlie the crucial role of the sense of smell during evolution. The interactions of ORs with volatile molecules in the cilia of olfactory sensory neurons cause protein conformation changes that lead ultimately to the production of action potentials that carry information to the brain. The structure of ORs is characterized by seven-transmembrane domain, the hallmark of all GPCRs. Structures have been experimentally solved for only 10% of GPCRs but no experimental structures are available for ORs. Computational methods appear as the only way to understand at the atomic level the mechanism of ligand binding and receptor activation for these proteins, even if the use of these methods is far from trivial. In fact, the sequence identity between members of the superfamily is often below 20% making the use of computational methods, especially homology modeling, a fascinating challenge. Our goal is to predict the ligand binding mode in the ORs and the effect of mutations that affect odor perception using homology modeling, possibly with inclusion of experimental data, and state of the art docking in combination with a hybrid molecular mechanics/coarse-grained (MM/CG) approach. In addition, we are developing a database compiling information about the known olfactory receptor-odorant pairs, RepOdor. This repository will facilitate a systematic analysis of the currently available data, with the aim of providing insights into the combinatorial code of smell and the therapeutic potential of odorants.
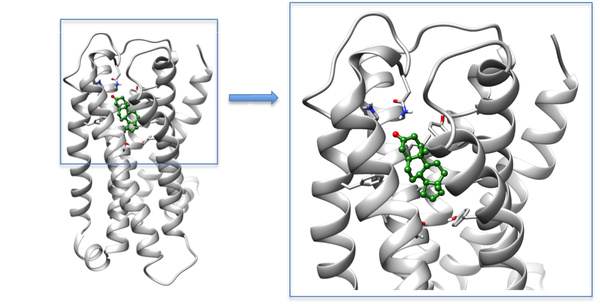
Figure 1 - Model of OR7D4 docked with its agonist androstenone.
References
- Leguebe M, Nguyen C, Capece L, Hoang Z, Giorgetti A, et al. (2012) Hybrid molecular mechanics/coarse-grained simulations for structural prediction of G-protein coupled receptor/ligand complexes. PLoS ONE 7: e47332.
- Biarnés X, Marchiori A, Giorgetti A, Lanzara C, Gasparin P, Carloni P, Born S, Brockhoff A, Behrens M, Meyerhof W (2012) Insights into the Binding of Phenyltiocarbamide (PTC) Agonist to Its Target Human TAS2R38 Bitter Receptor. PLoS ONE 5(8): p. e12394.
- Sandal M, Behrens M, Brockhoff A, Musiani F, Giorgetti A, Carloni P, Meyerhof W (2015) Evidence for a Transient Additional Ligand Binding Site in the TAS2R46 Bitter Taste Receptor. Journal of Chemical Theory and Computation 11(9): p. 4439-4449.
- Fierro F, Suku E, Alfonso-Prieto M, Giorgetti A, Cichon S, Carloni P (2017) Agonist binding to chemosensory receptors: a systematic bioinformatics analysis. Front. Mol. Biosci. 4: p. 63.
- Alfonso-Prieto M, Navarini L, Carloni P (2019) Understanding ligand binding to G-protein coupled receptors using multiscale simulations. Front. Mol. Biosci. 6: p. 29.
