Bitter taste receptors
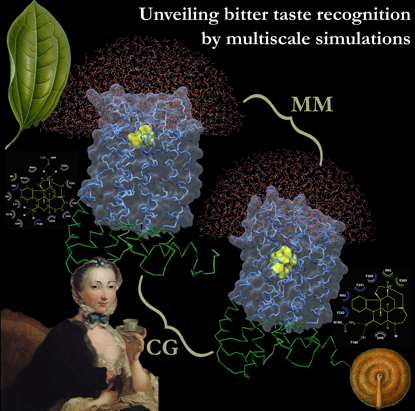
70% of the G-protein coupled receptors are activated by small molecules binding to their 7-transmembrane (7-TM) helix bundle. Some members of the class A branch have been suggested to feature not only an orthosteric agonist-binding site (corresponding to the proper binding site) but also a more extracellular or “vestibular” site, involved in ligand selectivity. In order to understand whether the presence of this “access control” is a more general feature of GPCRs, we performed hybrid molecular mechanics/coarse-grained (MM/CG) molecular dynamics on a human bitter taste receptor/agonist complex. Human bitter taste receptors subfamily (TAS2Rs) represents the second most populated GPCRs subfamily (25 members) and provides critical protection against ingestion of poisonous compounds. Experimental structural information on bitter taste receptors is lacking; therefore, the simulated complexes (TAS2R46/strychnine and TASR16/bitter sugars, some of the best characterized TAS2Rs/agonist complexes) have been generated using homology modeling and docking approaches. On one hand, multiscale simulations of TAS2R46 in complex with strychnine [1] revealed two cavities hosting the agonist; only considering both can one explain all the available experimental data. This two-step verification mechanism shares similarities with the one suggested for other class A GPCR members and it might be instrumental for recognizing the remarkably broad yet selective spectrum of agonists of TAS2R46. On the other, multiscale simulations of TAS2R16 in complex with different bitter sugars [2] uncovered a previously unknown dual binding mode. Such mechanism may offer a seamless way to fit different bitter sugars inside the binding cavity, similar to the strategy used by several carbohydrate-binding lectins. Our prediction is validated a posteriori by comparison with mutagenesis data and also rationalizes a wealth of structure-activity relationship data. On the basis of the obtained results, we plan a genome-wide TAS2Rs characterization extending the work made on broad spectrum TAS2R46 and group specific TAS2R16 to all the 25 human bitter taste receptors subfamily members. The objectives of the study include accurate prediction of binding poses for agonists, mapping the critical residues involved in the ligand binding site(s), and rationalization of the different specificities of different bitter taste receptors. Unraveling the molecular mechanism of activation of TAS2Rs may lead to an explanation of the effect of genetic variability on bitter taste sensing and other physiological processes. Moreover, a better understanding of the nature of interactions between ligands and the extracellular vestibule may provide a new opportunity for designing more subtype-specific ligands.
References
- Sandal M, Behrens M, Brockhoff A, Musiani F, Giorgetti A, Carloni P, Meyerhof W (2015) Evidence for a Transient Additional Ligand Binding Site in the TAS2R46 Bitter Taste Receptor. Journal of Chemical Theory and Computation 11(9): p. 4439-4449.
- Fierro F, Giorgetti A, Carloni P, Meyerhof W, Alfonso-Prieto M (2019) Dual binding mode of “bitter sugars” to their human bitter taste receptor target. Sci. Rep. 9: p. 8437.
