Soft wet-chemical synthesis of Ru-Sn nanoparticles from single-source ruthenocene-stannole precursors in an ionic liquid
December 2016
Microwave-induced decomposition of the single-source precursors of a neutral triple-decker ruthenocene (1) and an anionic ruthenocene (2) both bearing a stannole ligand in the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIm][BF4]) gives bimetallic ruthenium-tin nanoparticles. Particles of approximate composition Ru2Sn (from 1) and Ru3Sn7 (from 2) were obtained in this soft, wet-chemical synthesis.
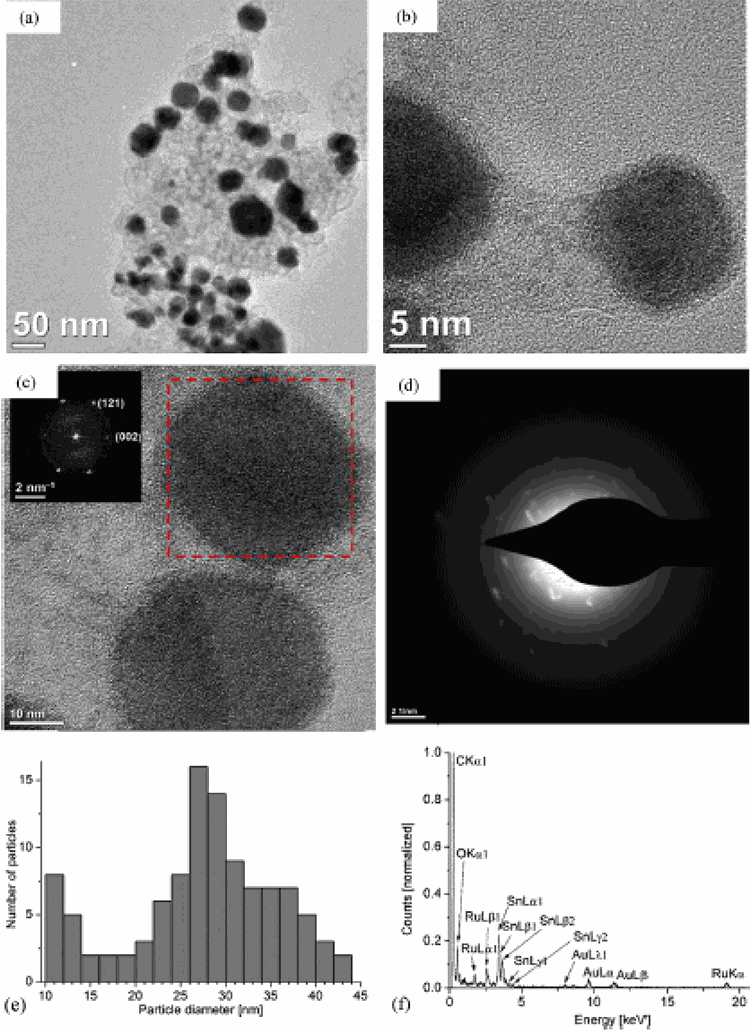
Transmission electron microscopy (TEM) showed the formation of 2–6 nm particles of Ru2Sn with its average elemental composition determined from energy dispersive X-ray spectroscopy (EDX). Crystalline Ru3Sn7 nanoparticles together with α-Sn nanoparticles of 10–50 nm (from TEM) were elucidated by positive matching of the powder-X-ray diffractogram to the Ru3Sn7 and α-Sn phase and the element composition from EDX.
Further reading:
Susann Wegner, Masaichi Saito, Juri Barthel and Christoph Janiak: Soft wet-chemical synthesis of Ru-Sn nanoparticles from single-source ruthenocene-stannole precursors in an ionic liquid,
Journal of Organometallic Chemistry 821 (2016) 192-196.
