CO2/N2 Separation on Highly Selective Carbon Nanofibers Investigated by Dynamic Gas Adsorption
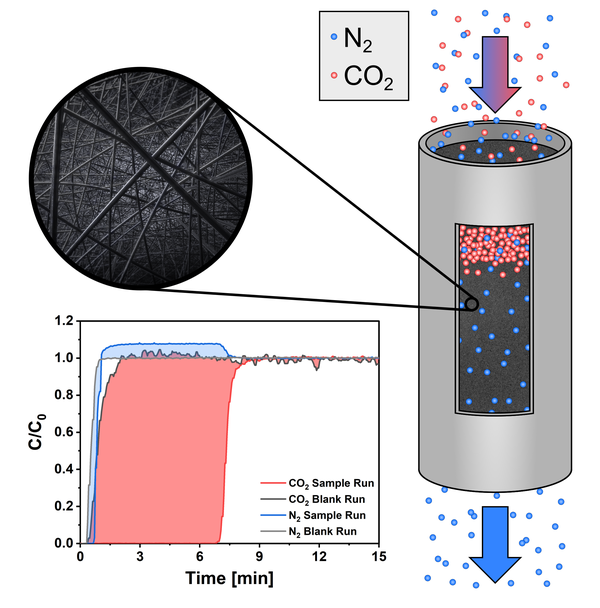
The vast emission of CO2 as a greenhouse gas is the main cause for the anthropogenic climate change. However, efficient separation technologies appear capable to reduce CO2 emissions by carbon capture and storage (CCS) or utilization (CCU). Currently, adsorption-based processes like pressure swing adsorption (PSA) are considered as promising and energy efficient approaches. A key component in these processes is the adsorbent material since its properties such as CO2 selectivity, ad- and desorption kinetics, long-term stability and regeneration conditions influence the performance of the separation process significantly. To evaluate the separation capability of a material on a laboratory scale, dynamic gas adsorption is a powerful tool as it offers the possibility to measure mixed gas adsorption under relevant conditions and allows to deduct important parameters such as the aforementioned CO2 selectivity from the resulting breakthrough curves.
In this work, Polyacrylonitrile (PAN) based carbon nanofibers (CNFs) are investigated for their CO2 separation capabilities using dynamic gas adsorption. The CNFs are prepared by electrospinning and subsequent carbonization at various temperatures ranging from 600 °C to 1000 °C. A thorough investigation of the CO2/N2 selectivity resulted in measured values of 53 to 106 at 1 bar and 25 °C on CNFs carbonized at 600 °C, 700 °C or 800 °C. Moreover, the selectivity increased with lower measurement temperatures and lower CO2 partial pressures reaching values up to 194. Further analysis revealed high long-term stability with no degradation over 300 cycles and fast adsorption kinetics for CNFs carbonized at 600 °C or 700 °C. These excellent properties make PAN-based CNFs carbonized at 600 °C or 700 °C promising candidates for the capture of CO2.
