Chemical stability of levoglucosan: An isotopic perspective
X. F. Sang, I. Gensch, B. Kammer, A. Khan, E. Kleist, W. Laumer, P. Schlag, S. H. Schmitt, J. Wildt, R. Zhao, E. L. Mungall, J. P. D. Abbatt, A. Kiendler-Scharr
Abstract
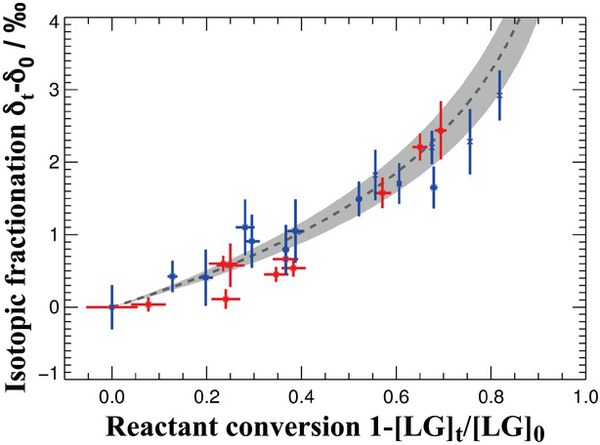
The chemical stability of levoglucosan was studied by exploring its isotopic fractionation during the oxidation by hydroxyl radicals. Aqueous solutions as well as mixed (NH4)2SO4-levoglucosan particles were exposed to OH. In both cases, samples experiencing different extents of processing were isotopically analyzed by Thermal Desorption-Gas Chromatography-Isotope Ratio Mass Spectrometry (TD-GC-IRMS). From the dependence of levoglucosan δ13C and concentration on the reaction extent, the kinetic isotope effect (KIE) of the OH oxidation reactions was determined to be 1.00187±0.00027 and 1.00229±0.00018, respectively. Both show good agreement within the uncertainty range. For the heterogeneous oxidation of particulate levoglucosan by gas-phase OH, a reaction rate constant of (2.67±0.03)·10−12 cm3 molecule−1S−1 was derived. The laboratory kinetic data, together with isotopic source and ambient observations, give information on the extent of aerosol chemical processing in the atmosphere.
