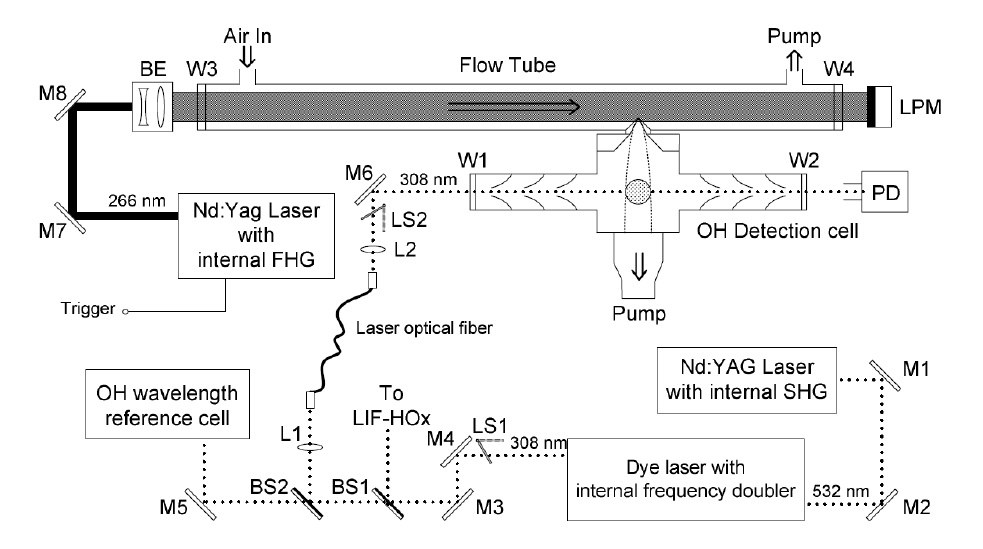
LIF - Laser induced fluorescence

Laser-induced fluorescence (LIF) technique is a very sensitive, analytic method applied for the detection of OH radicals. It makes use of specific, spectroscopic properties of OH in the UV and allows measuring concentrations with a temporal resolution of seconds to minutes. Ambient air is sampled through an inlet nozzle in a low pressure detection cell. OH radicals in the sampled air are excited by a high-frequency pulsed laser and the subsequent fluorescence is detected by photon counting. The intensity of the fluorescence light can be converted into an ambient radical concentration utilizing the instrument sensitivity which has to be determined in a separate calibration measurement using a well-characterized radical source. The limit of detection for atmospheric measurements is approximately 3 x 105 molecules/ cm3 at a temporal resolution of one minute. The high sensitivity of the instrument allowed measuring the natural fluctuation of OH in the atmosphere for the first time.
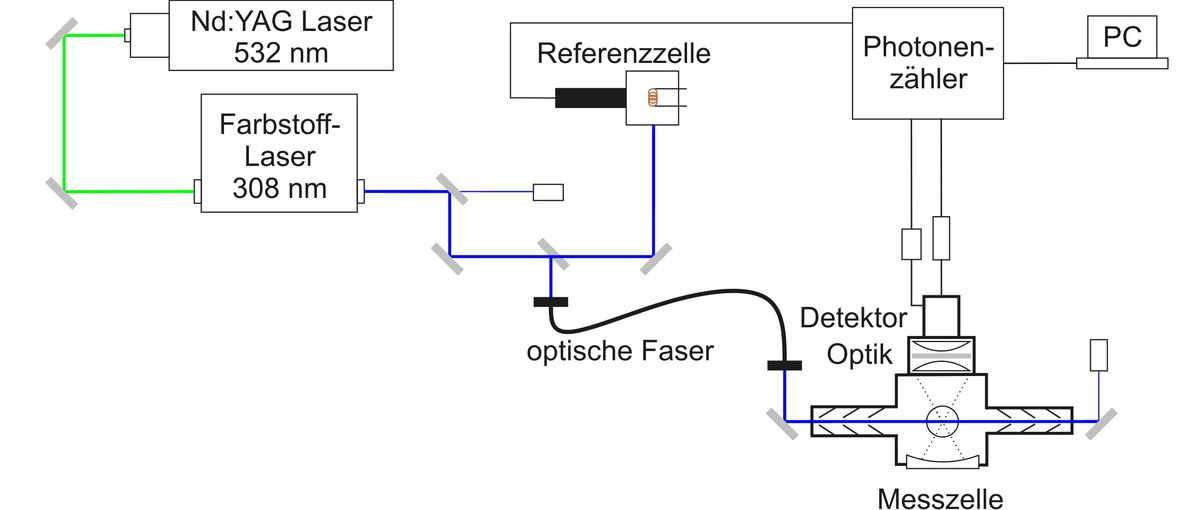
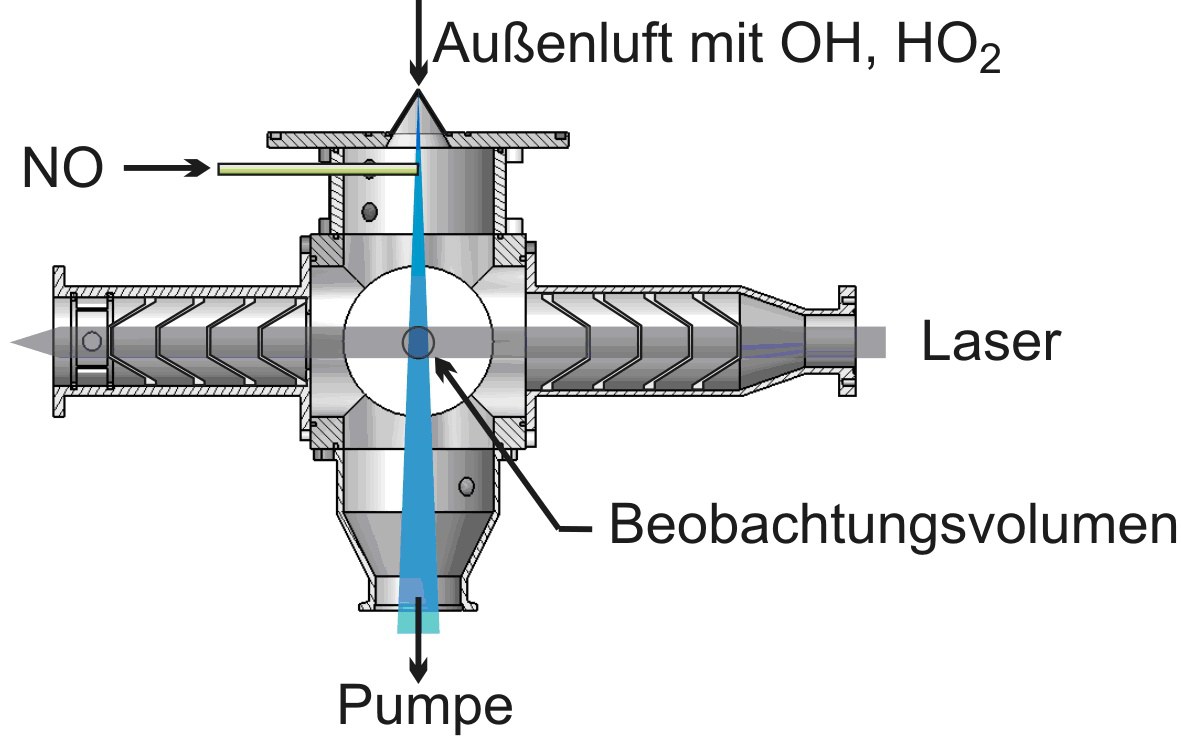
The LIF technique is also used for the measurement of atmospheric concentrations of HO2 radicals. HO2 is chemically converted to OH in its reaction with nitrogen monoxide (NO) in a second detection cell with subsequent detection of OH by laser-induced fluorescence, so that the sum of OH und HO2 (=HOX) is measured. The difference between OH and HOX measurement gives the atmospheric HO2 concentration. Simultaneous measurement of OH und HO2 allows quantification of chemical processes that are important for oxidation of pollutants in the atmosphere.
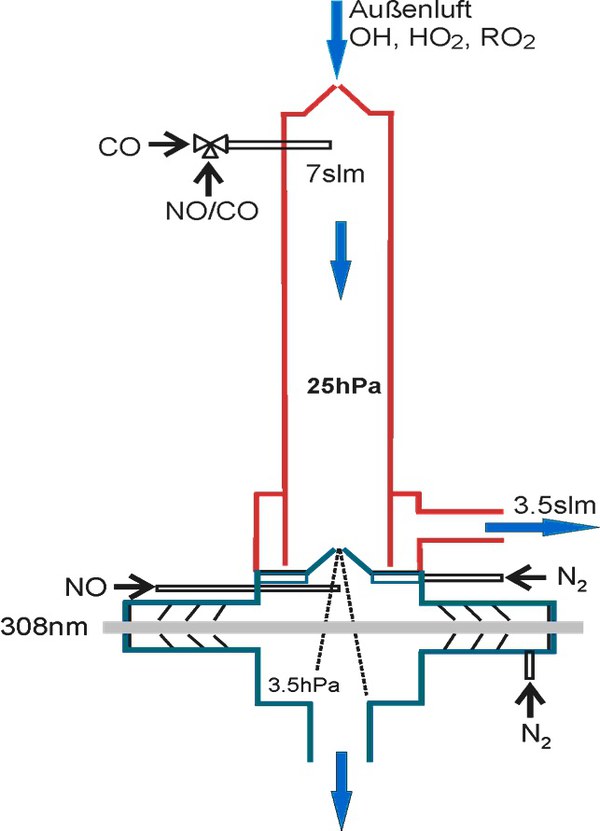
Furthermore, organic peroxy radicals (RO2) can be detected by the LIF technique using a similar conversion scheme as for HO2. This measurement channel consists of a conversion reactor that is mounted on top of an HO2 detection cell. First, RO2, HO2 und OH are chemically converted to HO2 in the conversion reactor at reduced pressure by adding NO and CO. The HO2 concentration is then measured in the detection cell by further increasing the NO concentration. RO2 concentrations can be calculated by subtracting OH and HO2 measured in the two other channels of the instrument.
Two LIF instruments are operated at ICE-3. Each instrument is equipped with measurement channels for concentrations of OH, HO2 and RO2, as well as the kinetic measurement of atmospheric OH lifetimes. One instrument is employed in ground-based field campaigns, on the Zeppelin or on the research aircraft HALO. The other instrument is permanently installed at the atmosphere simulation chamber SAPHIR.
The chemical lifetime of OH radicals in the atmosphere can be experimentally determined in addition to OH concentration measurements by another instrument. Ambient air is flown through a tube, in which a pulse of high OH concentration is produced, which is homogeneously distributed. The OH concentration is measured at the end of the tube by the LIF method. The temporal behavior of the concentration converts to the lifetime of OH, since OH reacts with trace gases in the sampled air after it has been produced by the short laser pulse.