GABARAP-Ligand-Interaction
Insight into the specifics of GABARAP ligands was first gained from phage display screening experiments. The majority of GABARAP-binding peptides contain at least one tryptophan residue. Since the side chain of tryptophan contains a rigid aromatic indole moiety, indole derivatives such as indole acetic acid (IAA) were used to characterize the binding properties of GABARAP by two-dimensional NMR spectroscopy. Here, two apolar regions on the surface of GABARAP with a distinct affinity for IAA were identified. These regions were named hydrophobic pocket 1 (HP1) and HP2.
Using X-ray crystallography, we were able to obtain structural information on the interactions of GABARAP with artificial as well as physiological ligands.
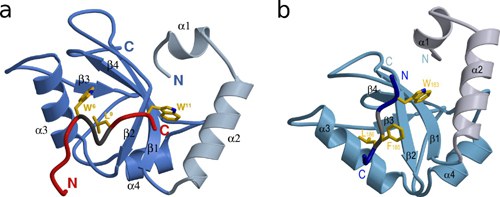
A GABARAP-binding peptide with the sequence DATYTWEHLAWP (so-called K1) was found several times in phage display screens. The three-dimensional structure of the GABARAP-K1 complex was determined by X-ray structural analysis (Figure 1a). This complex is stabilized by hydrophobic interactions between tryptophan residue W6 and leucine residue L9, which interact with HP2, and by the interaction of tryptophan residue W11 with HP1.
Calreticulin is a cellular interaction partner of GABARAP identified in our laboratory. Calreticulin is known for its function as a calcium-dependent chaperone in the endoplasmic reticulum. Although it is mostly localized in the ER lumen, its retrotranslocation back to the cytosol has also been demonstrated, making it available there for interaction with GABARAP. Indeed, colocalization studies revealed similar distribution patterns of both proteins and recombinant GABARAP can bind calreticulin from cell lysates.
In a subsequent study, different calreticulin fragments were investigated with respect to their interaction with GABARAP. SPR and NMR experiments showed that the peptide consisting of amino acid residues 178-188 of calreticulin (CRT178-188) contains the primary binding motif, and that this is essential for interaction with HP1 and HP2 of GABARAP. The three-dimensional structure of the GABARAP - CRT178-188 complex was also determined by X-ray structural analysis (Figure 1b). The central part of the peptide ligand forms hydrogen bonds to the β2-strand of GABARAP and can therefore be regarded as an intermolecular extension of the central β-sheet. Overall, the interaction is again dominated by hydrophobic contacts of amino acid residues W183, F185, and L186 to GABARAP.
