Octahedral PtNi nanoparticles
Fuel cell technology is of great importance for future energy conversion and storage applications. However, a major obstacle that currently limits the performance of technologically important proton exchange membrane (PEM) fuel cells is the sluggish oxygen reduction reaction (ORR) at fuel cell cathodes.
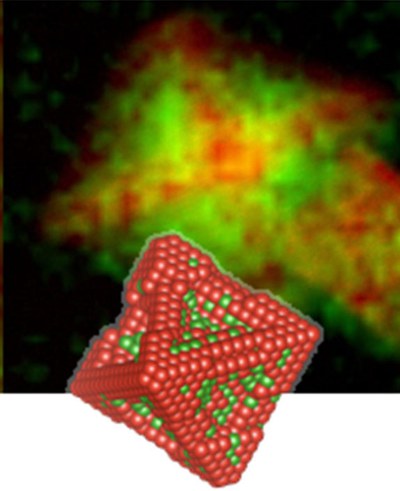
Because of their catalytically highly active (111) surfaces, bimetallic Pt-Ni octahedra are regarded as outstanding catalysts for the ORR. The elemental distribution of the Pt-alloy catalyst nanoparticles is of decisive importance for their activity and stability and requires atomic-scale structural and compositional analysis.
Using aberration-corrected high-resolution analytical electron microscopy, we have uncovered that compositional segregation in shaped Pt alloy nanoparticles, e.g. Pt-rich frames and Ni-rich facets results in complex corrosion of the nanoparticles and is a main reason for degradation of the catalysts during the ORR. Most importantly is therefore to understand and to control the mechanisms of formation and degradation in order to develop more active and stable novel nanoparticle catalysts.
Using the ‘PICO’ microscope at the Ernst-Ruska Centre, we have revealed an element-specific anisotropic growth mechanism of bimetallic nano-octahedra where compositional anisotropy couples to geometric anisotropy and rapid growth of Pt-rich hexapods along <100> directions precedes delayed deposition of Ni-rich phase at the concave {111} sites. This element-specific growth is the cause of compositional segregation and the main reason for the previously reported degradation and loss of activity for octahedral Pt-alloy nanoparticle catalysts.
Our current research is focused on the optimization and stabilization of the microstructure of the catalyst nanoparticles by suitable methods like surface doping or additional surface treatments by combining analytical electron microscopy with nanoparticle synthesis and electrochemistry. We have for instance shown that Rh-doped Pt-Ni octahedral nanoparticles possess high ORR activities combined with improved performance and shape stability compared to bimetallic Pt-Ni octahedral particles.
Scanning transmission electron microscopy (STEM) and energy dispersive X-ray analysis (EDX) show that the migration of Pt surface atoms is a primary origin of the octahedral shape loss for Pt-Ni nanoparticles. Using small amounts of Rh we were able to suppress the migration rate of platinum atoms and consequently suppress the octahedral shape loss of Pt-Ni nanoparticles.
For more details please refer to the papers:
DFG project: “Well-defined Nanoscale Shaped Pt Alloy Electrocatalysts: Synthesis, Electrochemical Analysis, and ex-situ/in-situ TEM Studies.“
(Marc Heggen)
C. Cui, L. Gan, M. Heggen, S. Rudi, P. Strasser, Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis, Nature Materials 12, 765–771 (2013)
L. Gan, C. Cui, M. Heggen, F. Dionigi, S. Rudi, P. Strasser, Element-specific anisotropic growth of shaped platinum alloy nanocrystals, Science 346, 1502 -1506 (2014)
M. Heggen, M. Gocyla, R. E. Dunin-Borkowski, The growth and degradation of binary and ternary octahedral Pt-Ni-based fuel cell catalyst nanoparticles studied using advanced transmission electron microscopy, Advances in Physics X 2, 281–301 (2017)
V. Beermann, M. Gocyla, E. Willinger, S. Rudi, M. Heggen, R. E. Dunin-Borkowski, M.-G. Willinger, P. Strasser,Rh-Doped Pt–Ni Octahedral Nanoparticles: Understanding the Correlation between Elemental Distribution, Oxygen Reduction Reaction, and Shape Stability, Nano Letters 16, 1719-1725 (2016)
N. Erini, V. Beermann, M. Gocyla, M. Gliech, M. Heggen, R. E. Dunin-Borkowski, and P. Strasser, The Effect of Surface Site Ensembles on the Activity and Selectivity of Ethanol Electrooxidation by Octahedral PtNiRh Nanoparticles, Angewandte Chemie 129, 6633 (2017)

Contact:
Dr. Marc Heggen
Phone: +49 2461 61-9479
E-Mail: m.heggen@fz-juelich.de
