IAS-2 Seminar: Quantification and Modeling of Spatial Biological Networks
Dmitry Fedosov
Philip Kollmannsberger
Institut für Biomedizinsche Physik, Heinrich Heine Universität Düsseldorf
Join us in person in Building 04.16, Room 2001
Abstract:
Quantification and Modeling of Spatial Biological Networks
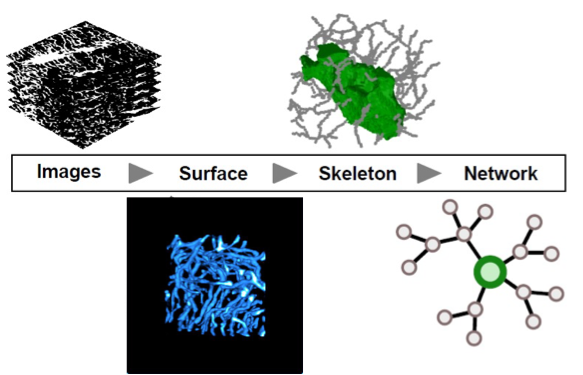
Spatial biological networks are abundant on all levels of life, from single cells to ecosystems. We develop and apply computational methods for automated image and network analysis, aiming towards a quantitative understanding of cellular interactions in tissues. One striking example is the osteocyte network in the bone tissue of most vertebrates. Osteocytes reside in a large, interconnected network of voids pervading the mineralized bone matrix. This osteocyte lacuno-canalicular network (OLCN) is believed to play important roles in mechanosensing, mineral homeostasis, and the mechanical properties of bone.
Quantitative measures enable insights into how the OLCN is organized regarding intercellular transport and communication. The cell network in slow-growing bone tissue from sheep is less connected but more efficiently organized compared to fast-growing bone tissue from mice. On the level of statistical topological properties, both network types are indistinguishable, highlighting that despite pronounced differences at the tissue level, the architecture of the osteocyte canalicular network at the subcellular level may be independent of species and bone type.
Computer simulations can help to understand how local cell behavior determines the resulting network architecture. I will present a computational framework based on directional statistics to model network formation in space and time under spatial constraints. Growth is described as a biased correlated random walk where direction and branching depend on the local environment represented as a 3D multilayer grid. We perform growth simulations of a dense network between osteocytes and compare the results to experimental data. Taken together, our results suggest a universal mechanism underlying the self-organization of individual cells into a large, interconnected network during bone formation.