Investigating NEF, the HIV Protein
Jülich researchers discover important role of previously disregarded protein
Jülich, 4 August 2017 – Around 37 million people around the globe are living with HIV. The illness associated with the infection, acquired immune deficiency syndrome (AIDS), can currently be treated with drugs – but is not yet curable. The human immunodeficiency virus (HIV) thus remains one of the greatest challenges for the public health system. The viral NEF (negative factor) protein is viewed as an important virulence factor contributing decisively to the course of the disease. Scientists at Jülich’s Institute of Complex Systems and at the Düsseldorf Institut für Physikalische Biologie
For a long time, NEF was viewed as unimportant in the development of the HIV disease, as attested by its name: negative factor, a small viral protein without enzymatic function. Depending on the virus isolate, it consists of 200–280 amino acids and is one of the less than 20 HIV proteins. We know today that NEF is one of the most important protein components of HIV. It manipulates human immune cells in a multitude of ways and thus contributes to the effective spreading and high virulence of HIV in humans.
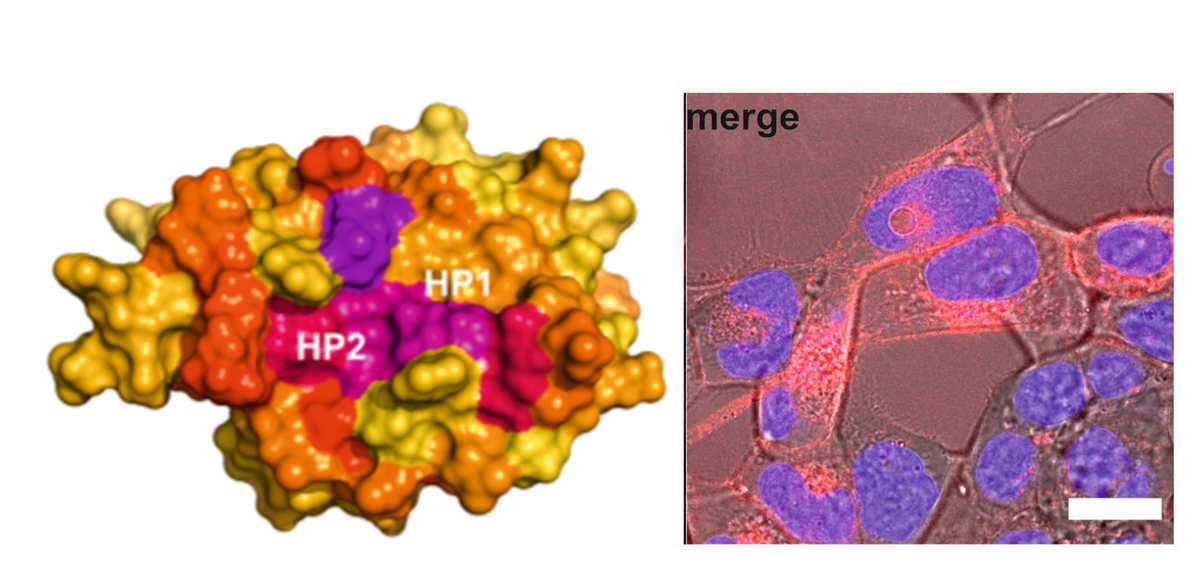
In order for NEF to execute some of its functions, it has to reach the inside of the surface of an infected cell. It is not yet understood how it is able to do so efficiently or what mechanisms are necessary. The Jülich working group headed by Silke Hoffmann from the Institute of Complex Systems – Structural Biochemistry (ICS-6) has now verified that NEF requires the host protein GABARAP or its close relatives GABARAPL1 and -L2 in order to dock to the cell membrane. "Only if NEF is localized at the plasma membrane can it initiate the downregulation of the immune system, for example by removing tiny surface receptors such as CD4 or MHC-I," explains Hoffmann.
How important NEF is in the development of the illness is reflected in patients who are infected with virus isolates in which the NEF protein structure is defective: "These people can carry HIV for very long times without the illness actually breaking out," says Hoffmann. For this reason, there is great scientific interest in decoding the various functions of NEF – a gigantic jigsaw puzzle. "We were all the more astonished when we found a human interaction partner, GABARAP, which we have been investigating at our institute for many years, and realized that if we remove it and its close relatives from our cell lines, then NEF can no longer reach the location in the cell that it needs to in order to be effective," explains Alexandra Boeske, who conducted the decisive experiments at ICS-6 and currently works at Heinrich Heine University Düsseldorf (HHU). Against the backdrop that the localization of NEF at the plasma membrane is so important for many effects of HIV pathogenesis, this is a crucial gain in knowledge.
At Jülich’s Biomolecular NMR Center, the scientists were able to identify the binding site for HIV-NEF on the molecular surface of GABARAP; the necessary 3D structure of GABARAP had previously been decoded there. Next, the researchers aim to cooperate with virologists to investigate whether infected cells really no longer downregulate immune receptors – i.e. the immune system – if GABARAP is not present. "This would verify in a biologically relevant system the significance we suspect GABARAP has in the process," says Hoffmann.
GABARAP itself is a member of a protein family which is involved in intracellular transport processes and autophagy, the process in which cells break down and recycle their own components, but which also serves to fight viruses and bacteria. In addition, cells appear to use parts of the autophagy process for the secretion of proteins. In this context, the researchers also observed that the lack of GABARAP not only prevents NEF from localizing at the cell membrane, but also inhibits the transport of the viral protein to the outside, into the extracellular space, where NEF is known to damage uninfected cells and thus also contributes decisively to pathogenesis. The structural and molecular biologists are planning to present the precise relation between autophagy, GABARAP, and NEF secretion in another publication in the near future. The project is also funded by the German Research Foundation (DFG) as part of the collaborative research centre SFB1208.
Original publication:
Alexandra Boeske, Melanie Schwarten, Peixiang Ma, Markus Tusche, Jessica Mötter,
Christina Möller, Philipp Neudecker, Silke Hoffmann und Dieter Willbold: Direct binding to GABARAP family members is essential for HIV-1 Nef plasma membrane localization.
Scientific Reports 7, Article number: 5979 (2017), DOI: 10.1038/s41598-017-06319-4
Further information:
Forschungszentrum Jülich, Institute of Complex Systems, Structural Biochemistry (ICS-6)
Contact:
Dr. Silke Hoffmann
Forschungszentrum Jülich, Institute of Complex Systems, Structural Biochemistry (ICS-6)
Tel.:+49 2461 61-9448
E-Mail: si.hoffmann@fz-juelich.de
Prof. Dieter Willbold
Heinrich-Heine-Universität Düsseldorf, Institut für Physikalische Biologie und Forschungszentrum Jülich, Institute of Complex Systems, Structural Biochemistry (ICS-6)
Tel. +49 2461 61-2100
E-Mail: d.willbold@fz-juelich.de
Press contact:
Erhard Zeiss
Press officer
Forschungszentrum Jülich
Tel.: +49 2461 61-1841
E-Mail: e.zeiss@fz-juelich.de
